Guilford, CT. – October 13, 2025. AbTCR T Cells Targeting GPC2 Show Potent Efficacy Against Low-Antigen Neuroblastoma.
A recent study published in Cell Reports Medicine highlights a collaborative effort between the National Institutes of Health (NIH), Eureka Therapeutics, and Spatomics LLC. The research demonstrates that antibody-γδ T cell receptors (AbTCRs) targeting the oncofetal antigen GPC2 can induce potent regression of neuroblastoma, even in tumors with very low antigen density.
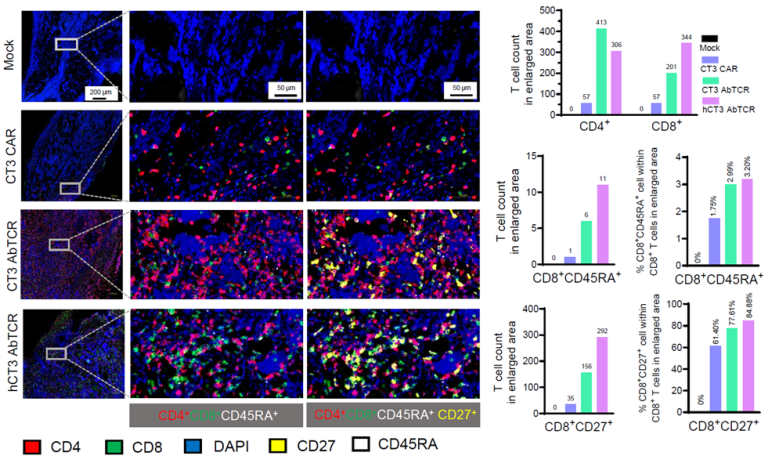
The team engineered AbTCR T cells using CT3 or humanized CT3 (hCT3) antibodies and found that these cells exhibited stronger cytotoxic activity and greater CD8⁺ T-cell infiltration than conventional CAR-T cells. These findings suggest AbTCR-based therapy as a promising approach for solid tumors that typically resist standard immunotherapies.
Spatomics’ CFP™ Multiplex Protein Detection Assay played a key role in the study by enabling high-resolution spatial profiling of immune cell infiltration and activation in tumor tissues. Using CFP™ cleavable tyramide signal amplification, researchers were able to visualize and quantify T-cell subsets such as CD45RA⁺, CD27⁺, and CD8⁺ within the tumor microenvironment, providing mechanistic insights into AbTCR function and efficacy.
Advantages of CFP™ Technology
- High sensitivity and precision for visualizing immune cell dynamics in FFPE tissues
- Tyramide-based signal amplification for detecting low-abundance targets
- Gentle fluorophore cleavage for multi-round, iterative staining without damaging tissue morphology
- Compatibility with standard automated IHC stainers such as Leica BOND Rx and Biocare Oncore Pro X
Together, these results underscore how Spatomics CFP™ reagents empower spatial immune profiling and accelerate next-generation cell therapy and tumor immunology research.

Read the full article →